Recent Aspects of Pulmonary Drug Delivery System - An Overview
Pulmonary delivery of drugs has become an attractive target in the health care industry as the lung is capable of absorbing pharmaceuticals either for local deposition or for systemic delivery. Half of all pharmaceuticals are not soluble in water, but are soluble in lipid. As the lung is able to absorb both water and oil into the tissue, this is not a limitation of pulmonary delivery. However, existing jet nebulizers cannot aerosolize viscous liquids and ultrasound nebulizers destroy drug emulsions, thereby greatly limiting the potential use of pulmonary drug delivery.
The whole 17 pages article is available for download here.
Current nebulizers also cannot effectively deliver peptide or protein based biotechnology drugs. Targeted drug delivery to the lungs has evolved to be one of the most widely inves¬tigated systemic or local drug delivery approaches. The use of drug delivery systems (DDS) for the treatment of pulmonary diseases is increasing because of their potential for localized topical therapy in the lungs. This route also makes it possible to deposit drugs more site-specific at high concentrations within the diseased lung thereby re¬ducing the overall amount of drug given to patients (10-20 % of the peroral quantity), as well as increasing local drug activity while reducing systemic side effects and first-pass metabolism.
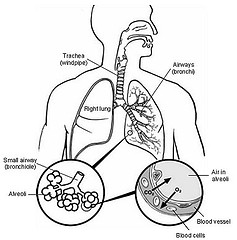
Pulmonary drug delivery systems have been used for decades to deliver drugs for treatment of respiratory disorders. The lungs provide a huge surface area of alveoli with rich capillary network which acts as an excellent absorbing surface for administration of drugs. Throughout the past several years, rapid onset of action and higher efficiency has been responsible for the success of pulmonary delivery system for symptomatic relief in treatment of asthma and chronic obstructive pulmonary disease (COPD). Research in the area of pulmonary drug delivery has gathered momentum in the last several years, with increased interest in using the lung as a means of delivering drugs systemically. Advances in device technology have led to the development of more efficient delivery systems capable of delivering larger doses and finer particles into the lung. As more efficient pulmonary delivery devices and sophisticated formulations become available, physicians and health professionals will have a choice of a wide variety of device and formulation combinations that will target specific cells or regions of the lung, avoid the lung's clearance mechanisms and be retained within the lung for longer periods. It is now recognized that it is not enough just to have inhalation therapy available for prescribing; physicians and other healthcare providers need a basic understanding of aerosol science, inhaled formulations, delivery devices, and bioequivalence of products to prescribe these therapies optimally. The recent approval of inhaled insulin stands as a major advancement not only in the field of diabetes treatment, but also for pulmonary delivery of macromolecules and systemically acting drugs.1 Although perhaps not readily apparent, the significance and impact of this accomplishment is far reaching, as many of the technical and perceived barriers obstructing the development of pulmonary drug delivery of systemically acting drugs have been dismantled by this achievement. Among the frequently cited advantages of pulmonary drug delivery are targeted delivery for improved efficacy and reduced incidence of unwanted systemic side effects, a large surface area for absorption, a relatively thin alveolar epithelium permitting rapid absorption, absence of first-pass metabolism, rapid onset of action, and enhanced bioavailability. For these reasons, systemically acting small and large molecules are attractive candidates for pulmonary delivery when more traditional routes are either impractical or unacceptable. As follows, many commercial and academic groups have sought to develop inhalation aerosols that demonstrate these advantages for clinically relevant disease states. Numerous carriers and particulate systems for pulmonary delivery have been designed to directly address these delivery issues. Until recently, these systems may have been seen as high risk investments with many assumed barriers to achieving commercial success. Indeed, many such technologies are distant from the regulatory approval stage, but there is now renewed confidence that they will find eventual clinical application for respiratory drug delivery. This article highlights some classes of carrier systems currently under investigation for specifically overcoming the hurdles facing the next generation of pulmonary drug delivery technologies. The development of controlled release formulations for inhalable drugs has been widely investigated since several years. Nonetheless, no controlled release product for pulmonary application is currently on the market.
The whole 17 pages article is available for download here.